August 16, 2024
2024 The Very Best Bpc-157 Powder Provider Pdf
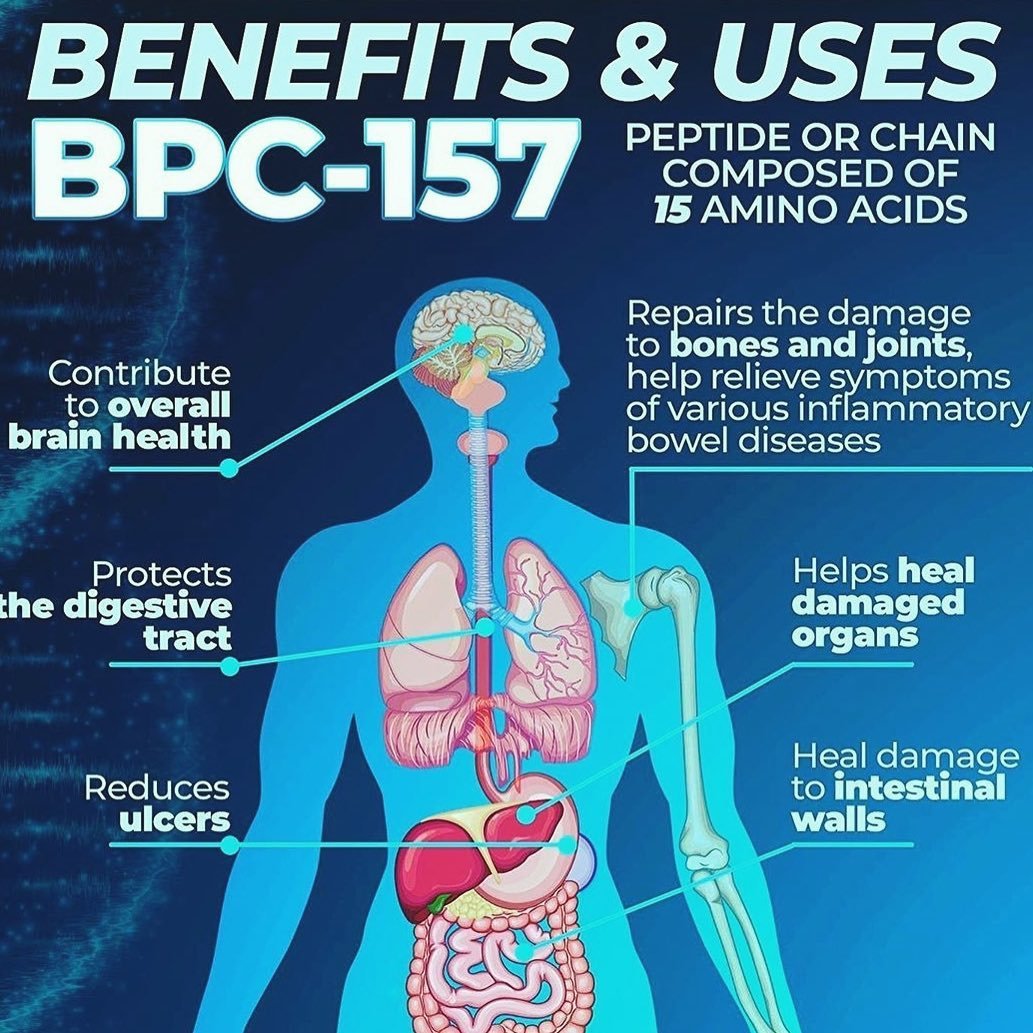
2024 The Best Bpc-157 Powder Supplier Pdf We focused on the application of the steady gastric pentadecapeptide BPC 157 [1,2,3,4,5,6,7,8,9,10,11] to boost the outcomes of spinal cord injury in rats. The theory of cell biology in injury recovery stressed that endothelial cells, fibroblasts, and keratinocytes might contribute to the expansion stage in the injury healing procedure. To confirm the theory, the MTT assay and cell cycle circulation were made use of to examine the impact of BPC-157 on cell proliferation. Previous studies have found that BPC-157 did not apply a direct result in regards to accelerating the cell spreading of cultured tendon fibroblasts,42 however our results recommended that BPC-157 modulates the cell viability and impacts HUVEC cell cycle leave in G0/G1 stage. To take a look at the impact of BPC-157 on angiogenesis in vitro, tube development assay was executed as defined formerly.28 In this assay, we utilized two study procedures. In the very first procedure, development factor-reduced matrigel was pipetted right into prechilled 24-well plates (150 mL matrigel per well) and polymerized for 45 mins at 37 ° C.BPC-157 and TB-500: Inflammation, Tissue Damage, and More - The Portugal News
BPC-157 and TB-500: Inflammation, Tissue Damage, and More.
Posted: Tue, 19 Sep 2023 07:00:00 GMT [source]
How Bpc-157 Assists In Accelerated Recovery
- No recognizable distinction in the plasma concentration of BPC157 was found between male and women pet dogs.
- Moreover, extreme heart congestion, subendocardial infarction, kidney hemorrhage, brain edema, hemorrhage, and neural damages were protected against.
- For future scientific applications, we had actually formerly established a solid-phase synthesis process for BPC157, verified its organic task in various wound designs, and finished preclinical security assessments.
- More surprisingly, BPC-157 is extremely steady and resistant to hydrolysis or enzyme food digestion, even in the stomach juice.
- The cells circulation results showed that the radioactivity strength in a lot of cells came to a head 1 h after administration, which was somewhat later than the peak time of the total radioactivity concentration in plasma (0.167 h).
What Are The Major Advantages Of Utilizing Bpc-157?
The reliable dose of BPC157 for the treatment of numerous injuries in mice, rats, and bunnies ranges from 6 to 50 μg/ kg (Huang et al., 2015; Mota et al., 2018; Sikiric et al., 2018). Our suggested medical dose of BPC157 was 200 µg/ person/day, and its equal dosage in rats was 20 μg/ kg (transformed based on body area). For that reason, we performed pharmacokinetic researches of BPC157 in rats following a single intravenous (IV) administration of 20 μg/ kg, solitary intramuscular (IM) administration of doses 20, 100, or 500 μg/ kg, and repeated IM administrations of 100 μg/ kg of BPC157 for seven successive days.Bpc-157 & Tb-4 & Ipamorelin Blend
The peptide's communication with the body is a dance of accuracy, convincing cells to discard sleepiness in favor of action. By stimulating the signaling pathways that moderate development and fixing, BPC-157 imparts a passion for recuperation at the fundamental degree of biological structure. Incredibly, BPC-157 beckons capillary to unfurl their network extra rapidly, consequently nurturing harmed areas with a rejuvenating circulation. This angiogenic impact drives a waterfall of fixing, breathing life right into cells that previously languished in recovery's slow-moving welcome. The detailed ballet of cellular regrowth and fixing discovers a powerful companion in BPC-157, a peptide whose healing touch murmurs promises of swifter recovery and restoration.As we transform our focus to the compound's regenerative impacts on various cells types, it comes to be clear that recognizing its interaction at the cellular level could reinvent our technique to recovery. Realizing the nuances of exactly how BPC-157 boosts all-natural healing procedures bids a much deeper appreciation for the body's natural strength and capacity for self-healing. No new metabolites were located in pee, bile, and fecal examples other than the six components found in the plasma. In the blended pee samples gathered from 0 to 8 h, the web content of [3H] proline (M1), the primary metabolite, was greater, representing 13.9% (woman) and 11.7% (male) of the overall radioactivity. In combined urine examples collected in between 8 and 72 h, the percentage of tritium water was greater, making up 69.5% (female) and 75.3% (man) of the total radioactivity, and [3H] proline (M1) accounted for 3.11% (woman) and 4.17% (man) of the total radioactivity (Figure Informative post 5B). The complete radioactivity discharging in combined bile samples gathered between 0 and 72 h was reduced, and tritium water was mainly spotted, representing 91.2% (ladies) and 91.0% (males) of the example. Cells were collected and healthy proteins were removed making use of cell lysis barrier supplemented with 0.3% phenylmethylsulfonyl fluoride and proteinase and phosphatase preventions. Proteins were divided by salt dodecyl sulfate polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membranes (Millipore, Bedford, MA, U.S.A.). After cleaning three times with TBST (Tris-buffered saline supplemented with 0.1% Tween-20), the examples were bred for 1 hour at space temperature level with an additional antibody. Bound antibodies were spotted using the enhanced chemiluminescent substratum (ECL, Pierce, Rockford, IL, USA).Is BPC 157 safe?
These researches have not revealed clear poisoning or negative side effects. Nonetheless, the significant worry about BPC 157 is the lack of substantial evidence confirming its safety in people. This is especially critical given its possible impact on numerous mobile signaling pathways, which can position serious dangers.
Social Links
