September 5, 2024
Excessive Weight Medicines In Advancement Pmc
Anti-obesity Medication Discovery: Developments And Obstacles Nature Assesses Drug Exploration Restorative passion has been spurred by observations in rodents, where neutralization of acyl-ghrelin246, restraint of ghrelin O-acyltransferase (GOAT) as the activating fatty acylation enzyme247 or direct antagonism of GHSR248 have demonstrated decreases in body weight and food intake. Obesity is a quickly expanding disease that results from an inequality betweenfood consumption and power expense. However, treatment of excessive weight is hamperedby organic forces that resist maintenance of fat burning. The length of drugtreatment needed was believed to have to do with 12 weeks, the https://ewr1.vultrobjects.com/pharma-warehousing/Drug-recalls/product-pricing/tesofensine-an-unique-antiobesity-medicine.html length of time needed tobreak a bad routine or find out to ride a bicycle without training wheels. The unfavorable stomach impacts and acute tachycardia induced by GLP1R agonists precludes accomplishing the topmost efficacy that might be attained through activation of GLP1R signaling.What is the strongest fat loss drug?
What is the greatest weight loss prescription drug? The quantity of fat burning feasible with semaglutide, according to scientific studies, is significant. A 2022 study of 175 people showed 5.9% weight loss at three months and 10.9% at 6 months.
Negative Events
The various other evaluation concluded thatphentermine-topiramate is cost-effective, but that final thought rests onthe degree to which advantages are maintained post-medication cessation and thatfurther research studies are suggested [68] About the SURMOUNT medical trial programThe SURMOUNT phase 3 global clinical development program for tirzepatide in persistent weight monitoring started in late 2019 and has actually signed up more than 5,000 individuals with obesity or overweight across 6 registration studies, 4 of which are worldwide studies. SURMOUNT-1 and SURMOUNT-2 were submitted to the FDA and demonstrated tirzepatide dramatically decreased body weight compared with sugar pill in people coping with excessive weight or overweight, with or without kind 2 diabetes. In December 2018, Saniona announced statistically and medically considerable weight-loss for its serotonin-- noradrenaline-- dopamine reuptake inhibitor NS 2330 (tesofensine) (now Tesomet) in its stage III Viking study for treating excessive weight. Repetitive rodent screening mainly using diet-induced obese computer mice and rats has been the main screen to evaluate body weight decreasing. Hereditary models and, much more so, engineered mice where certain receptors have been deleted, and significantly so in a target-specific way, have shown of vital worth to examination of device of action. A number of various other peptide and small-molecule GLP1R agonists are presently in medical advancement, including formulations created for oral administration. An additional oral GLP1R agonist (GLPR-NPA) is presently in phase II clinical trials at Eli Lilly (Table 2) (see Relevant web links).- Our information suggest that tesofensine in rats did not harm sweetness detection or affect its palatability.
- Professional application will proceed and concentrate on relative efficacy and safety, which is hard to refer when best-in-class prospects are at the same time quickly progressing and not promptly easily accessible for straight comparative clinical study125.
- Antibodies developed with a minimal regularity in liraglutide-treated topics than in those dealt with by exenatide, likely because of its greater structural resemblance with human GLP-1 (97 vs. 52%).
- As discussed formerly in area 2.3, an adverse effects brought on by thenon-specific serotonin agonists, fenfluramine and dexfenfluramine, was heartvalve lesions, because of stimulation of the outer serotonin 2B receptor.
- Tesofensine Peptide might have different results on different people, yet it's ideal combined with a lowered calorie consumption and normal workout.
Attending To Prospective Adverse Effects And Security Considerations
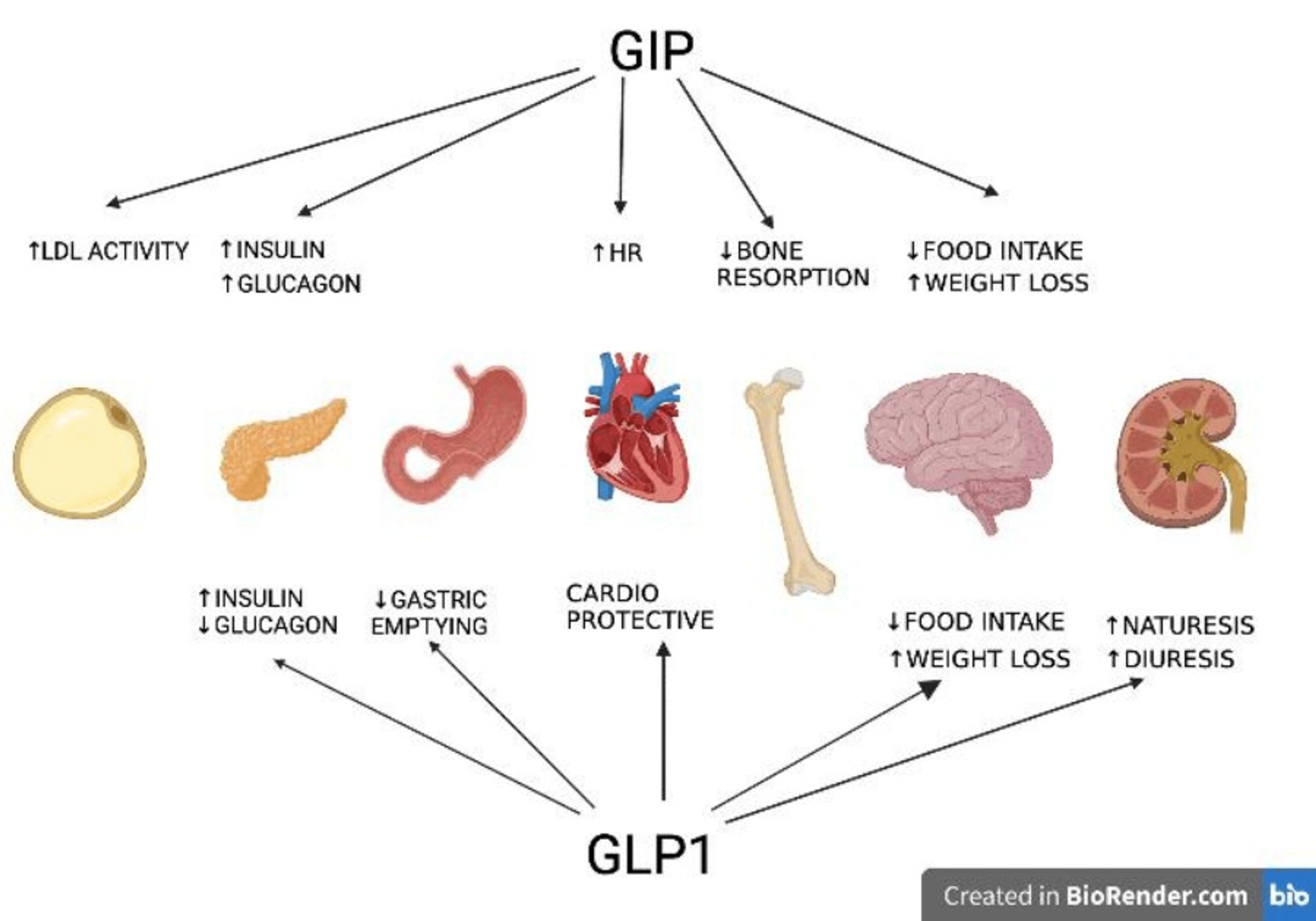
GABA release from AgRP/NPY estimates to extrahypothalamic nerve cells, in the parabrachial core, also plays a role in the excitement of food consumption (24 ). As well as boosting feeding, activation of NPY results in energy preservation by lowering the metabolic task of brownish fat in a manner paradoxical to that seen with policy of thermogenesis by POMC, by downregulation of understanding outflow from the locus coeruleus (25 ). Liraglutide (Victoza ® )is a glucagon-like peptide 1 (GLP-1) agonist that was accepted in 2010 for the treatment of T2DM; the suggested dose is subcutaneous (SC) administration of 1.8 mg everyday [50] The greater dose (3.0 mg SC everyday) of liraglutide (Saxenda ®) was approved by the FDA in 2014 and the EMA in 2015 for lasting weight administration. A. Rats were trained to lick a central spout that dispensed the stimulus a drop of water or services of sucrose. Upper panel shows the variety of tests, and the lower panel the proper performance across the standard, tesofensine treatment, and post-tesofensine days. Dose-dependent unfavorable stomach impacts were observed with tesofensine in the professional tests along with rises in blood pressure and heart. However, at the expected therapeutic dosage of 0.5 mg, discontinuations for unfavorable effects with tesofensine resembled placebo (8%). Unquestionably, the scientific outcomes with tirzepatide have actually caught fantastic interest and fuelled rate of interest in GIP-based double agonists and other combinatorial approaches. The scenario shows up to exhibit that despite the massive development in our molecular understanding of weight problems, we remain reasonably primitive in referring in vivo efficacy to system. It continues to be to be demonstrated in mechanistic detail how GIPR agonism acts as the basis for the enhanced efficacy of tirzepatide about dulaglutide. 

Social Links