September 5, 2024
Weight Problems Medications In Development Pmc
Component Three Next Generation Excessive Weight Treatments Undoubtedly, side effects have actually been a significant interest in all currently readily available anti-obesity drugs, as epitomised by the current withdrawal of Acomplia (rimonabant) from the European market. In the TIPO-4 trial, a 48-week open-label expansion to the TIPO-1 trial, preliminary results suggest that weight loss with tesofensine is maintained. After an initial eight-week washout duration, people proceeding with 0.5 mg tesofensine accomplished an overall mean weight management of 13-- 14kg at 24 weeks. In the growth of anti-obesity medicine various healing targets have actually been identified. They consist of serotonin and noradrenaline reuptake inhibitors (supposed anorectic agents), lipase inhibitors, b3-adrenoreceptor agonists, leptin agonists and melanocortin-3 agonists to name a few. Given the power of the strategy, multi-agonism treatment has actually been continuously used in preclinical treatment of obesity, normally yet not solely in mix with some form of GLP1 agonism.- After a preliminary eight-week washout duration, people continuing with 0.5 mg tesofensine accomplished a complete mean weight loss of 13-- 14kg at 24 weeks.
- Next-generation multi-omics have actually supplied some unique targets, but, generally, quickly evolving enabling technologies have been better in identifying preclinical system of action than in discovery of medically effective drug prospects.
- From 1967-- 1968,. the prevalenceof main lung high blood pressure was 20-fold more than it was in the periodfrom 1955-- 1966 in those nations.
- Diethylpropion is the preferred amphetamine-relatedanti-obesity medication in Brazil, as phentermine remains in the United States.Diethylpropion is to be made use of with caution listed below the age of 12 years and inpeople with epilepsy due to the initiation of seizures in people withepilepsy.
- Consequently, the growth of mitochondria-specific and more secure uncoupling agents appropriate for human usage may yet cause an effective and differentiated approach to dealing with these diseases263.
- Current advances, including raised understanding of the molecular intestine-- mind interaction, are inspiring the quest of next-generation AOMs that appear with the ability of safely attaining considerable and sustained body weight reduction.
0 Past Centrally Acting Anti-obesity Drugs
When https://seoneodev.blob.core.windows.net/pharma-tech/medical-devices/product/new-antiobesity-medicine-tesofensine.html taking into consideration drugs such as tesofensine vs semaglutide, it's vital to take into consideration the potential negative effects, among which could be sex-related disorder. While these medicines are mainly utilized for weight administration, they may have varying effect on sex-related health and wellness. In the context of weight monitoring medicines, Tesofensine and Semaglutide stand for two encouraging prospects. Both have actually revealed prospective in decreasing body weight, yet their effect on cardiovascular parameters are noteworthy.What is the future of obesity?
By 2030, almost half of U.S. grownups will certainly be overweight, consisting of the almost 1 in 4 who will have severe weight problems. The weight problems rate will certainly surpass 50% in 29 states.
Evommune Enrols Initially Subject In Persistent Inducible Urticaria Therapy Test
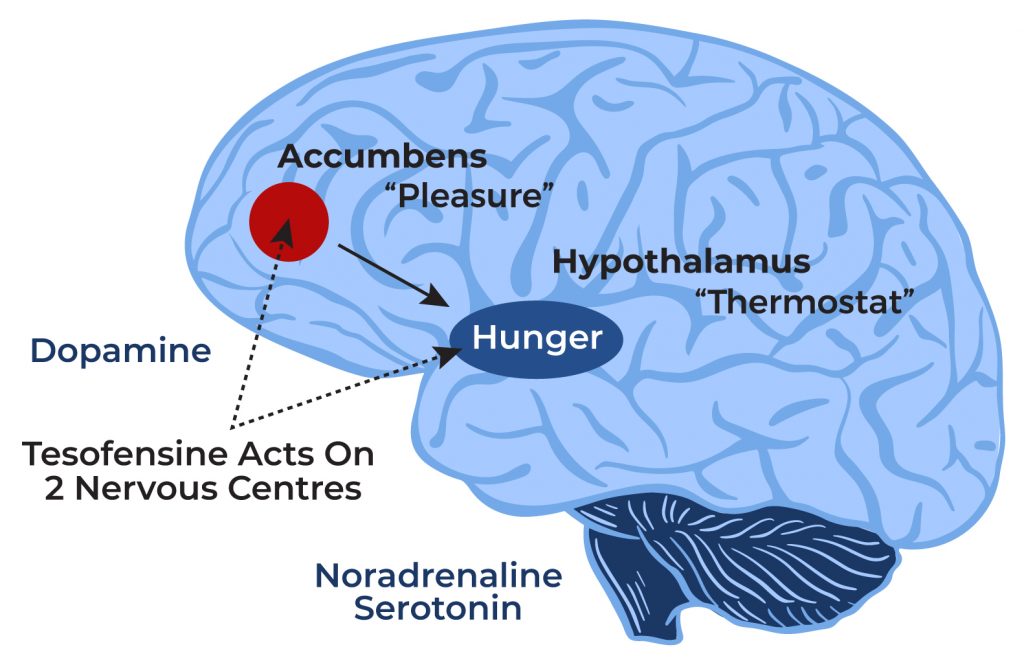
Tesofensinetreatment stabilized the dopamine degrees in the DIO rats, yet had no effect onthe chow-fed animals, suggesting that the anti-obesity results of tesofensineare due, a minimum of partly, to favorable inflection of central dopaminergicactivity [119] Because the significant damaging events resulting in discontinuation in theproof-of-concept trial were nausea or vomiting and throwing up attributable to naltrexone, a24-week phase II test examined three dosages of naltrexone with bupropion tofind one of the most tolerable dosage with enough efficiency. The trial randomized 419obese subjects to bupropion alone 400 mg/d, 3 mix dosages ofnaltrexone/bupropion (NB) with naltrexone at 16 mg/d, 32 mg/d, or 48 mg andbupropion 400 mg/d, or sugar pill [38] Theplacebo deducted weight management was biggest (4.65% of body weight) in the NB 32mg/d group by last observation continued (LOCF) evaluation because of higherdrop outs in the NB 48 mg/d group from nausea and vomiting [38] In a sub-study of this test, overall and visceralfat was determined by dual power x-ray absorptiometry (DXA) in a subset of 107participants. In the eighty topics that finished the sub-study, there was agreater decrease in total body fat (NB 14% vs. sugar pill 4%) and natural fat (NB15% vs. 4.6%) in the NB mix group contrasted to placebo or bupropion alone [39]Efficacy And Security Of The Weight-loss Medication Rimonabant: A Meta-analysis Of Randomised Tests
Consequently, we identified the tesofensine-induced stereotypy effects compared to phentermine, an amphetamine congener that worked as a favorable control. To evaluate stereotypic behavior, we made use of DeepLabCut, a markerless position estimation device based on transfer discovering with deep neural networks [34] We educated the network to spot a rat's nose, forelimbs, and tail base from a bottom-view videotaped session (see S1 Video). Despite there being no evidence of abuse, sibutramine was categorized in DEA routine IV as a result of architectural similaritieswith amphetamine [28] The rise inpulse and blood pressure were of problem to the regulatory authorities, and contingent onapproval, the sponsor agreed to do a cardio security study. That research study, called the SCOUT research study, registered topics with diabetes mellitus and heart problem, problems for which the drug was not accepted. All subjects, consisting of thosewho did not experience weight-loss, were kept on the medicine which would certainly not havebeen performed in regular technique. Individuals in the SCOUT trial revealed a 16% boost in cardiovascular endpoints like cardiac arrest, stroke and death [29] 
Social Links