
August 27, 2024
Brain-gut Axis And Pentadecapeptide Bpc 157: Academic And Sensible Implications
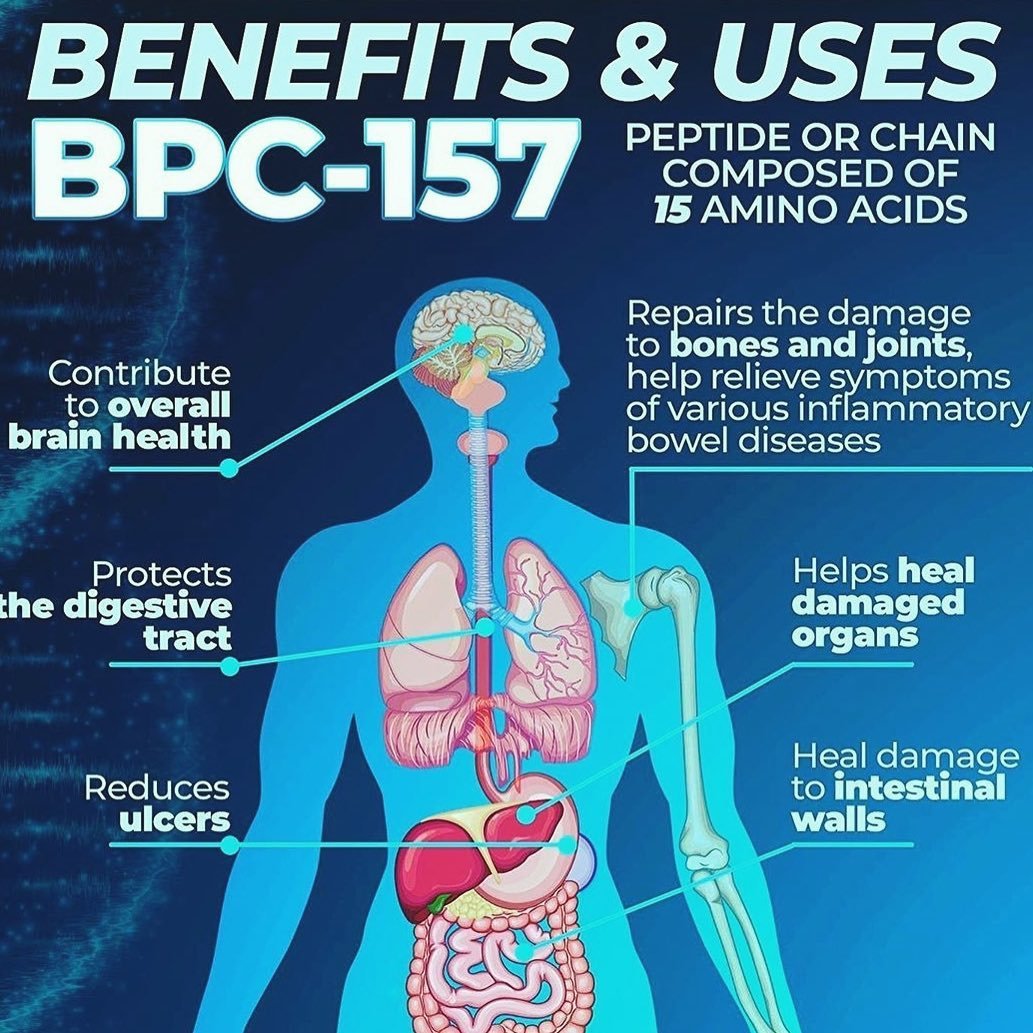
Steady Stomach Pentadecapeptide Bpc 157 Therapy For Key Stomach Area Syndrome In Rats Basically, BPC-157 boosts and maximizes the body's natural recovery and protective mechanisms. The anti-inflammatory homes of BPC-157 might aid reduce neuroinflammation, which is linked in numerous emotional and neurological problems, consisting of clinical depression, anxiousness, and neurodegenerative diseases. Participants likewise reach send concerns for AMA episodes, plus accessibility to unique bonus material. Nevertheless, there is proof that BPC-157 is being illegally included in some wellness and anti-aging treatments and products. Based upon current human research studies, BPC-157 can be safely utilized for 4 weeks complied with by a two-week break.How To Blend Bpc 157
Direct relationships were observed in between AUC0-- t and BPC157 dosages, along with in between Cmax and BPC157 doses (Figures 2D, E). The absolute bioavailability observed after IM management of each dosage in canines was 45.27%, 47.64%, and 50.56%, respectively. After duplicated IM administration of BPC157 at 30 μg/ kg for 7 consecutive days, the plasma concentration versus time contour resembled that observed after a solitary IM shot of 30 μg/ kg https://biopharma-innovations.b-cdn.net/biopharma-innovations/regenerative-medicine/2024-the-best-bpc-157-powder-supplier.html (Number 2C). Nevertheless, the pharmacokinetic criteria after repeated IM administration changed slightly contrasted to those observed after a solitary IM shot, with a tiny decline in Cmax and t1/2 and a rise in Tmax.Is Bpc-157 Secure?
- The proximal side of the esophageal laceration, or distal side of the duodenal incision, was ligated to prevent regurgitation [17,18,20-23]
- A camera attached to a VMS-004 Discovery Deluxe USB microscope (Veho, USA).
- These research studies suggest that BPC-157 might have anxiolytic and antidepressant effects, potentially because of its effect on neurotransmitter systems and swelling.
- Whichever method you decide to use BPC 157, it is essential to adhere to the correct dose directions.
- Nevertheless, it is very important to consult with your medical professional to guarantee compatibility and reduce the risk of negative communications.
What's The Existing Position Of The Fda On Bpc-157?
In rats that underwent esophagogastric anastomosis and L-NAME treatment, the final decline of stress within the esophagus at the website of anastomosis on day 4 happens simply prior to fatality. Here, additionally, we need to think dysfunction of the nitrergic pathway; for example, excision-immediate hefty loss of endothelium cells from the vascular wall results in a reduced NO-production capacity [61], which has various action for the damaged cells stability. We recognized curative treatment of esophagogastric anastomosis in rats with stable stomach pentadecapeptide BPC 157 (an anti-ulcer peptide secure in human stomach juice), as a novel moderator of Robert's cytoprotection that was effective in the whole intestinal tract, which was initially tested in clinical tests for ulcerative colitis and multiple sclerosis [1-7] The amplitude, polyphasic changes, and the proximal and distal CMAP latencies were taped, and the nerve conduction velocity was computed according to previous studies [41, 43] Histological assessment of skin sections with HE and Masson tarnishing offered understandings right into the morphology of skin layers and collagen degree during the healing procedure (Figure 2). Compared with model control, BPC-157-treated teams revealed a significant recovery response comparable to that of the bFGF-treated team. In the design control group, the granulation cells created were hypocellular and covered by a thin premature epithelium. It was plainly visible that the epidermal and subepidermal layers were well organized in the BPC-157- and bFGF-treated groups. On top of that, the BPC-157- and bFGF-treated teams showed far better granulation tissue development, reepithelialization, and facial makeover, when compared to the model control team, on the 18th day blog post wounding. The prototype medicine could not be discovered 4 h after management, and its removal half-life was much less than 30 minutes. BPC157 showed straight pharmacokinetic features in rats at the speculative dose. A new NO-system phenomenon, secure stomach pentadecapeptide BPC 157, together with NOS-blockade, L-NAME, and NOS-substrate L-arginine application [1], would favorably define esophagogastric anastomosis healing, esophagitis and stomach defect healing, along with rescue the "sphincter" stress at the site of anastomosis while preserving the pyloric sphincter stress. These approaches need to be made use of to counteract the regularly hazardous program after esophagogastric anastomosis creation. Furthermore, for a brand-new NO-system phenomenon, steady gastric pentadecapeptide BPC 157, along with NOS-blockade, L-NAME, and NOS-substrate L-arginine application [1], would favorably define esophagogastric anastomosis recovery, esophagitis and gastric problem recovery, in addition to rescue the "sphincter" stress at the website of anastomosis while preserving the pyloric sphincter pressure. In the rats that went through esophagogastric anastomosis, the particular factor of BPC 157 performance entailing both anastomosis healing and sphincter rescue was the realized anastomosis development already in controls that at the very least partly saved the sphincter function at the website of anastomosis, while pressure in the pyloric sphincter remains continuously reduced.Stable Gastric Pentadecapeptide BPC 157 Therapy for Primary Abdominal Compartment Syndrome in Rats - Frontiers
Stable Gastric Pentadecapeptide BPC 157 Therapy for Primary Abdominal Compartment Syndrome in Rats.
Posted: Sun, 12 Dec 2021 08:00:00 GMT [source]
Why is BPC outlawed?
The FDA cites & #x 201c; risk for immunogenicity, peptide-related pollutants, and minimal safety-related details & #x 201d; as factors for the BPC-157 ban. BPC-157 is still readily available as a dental pill.

Social Links